David Geier's Vaccine Study Review: An HHS Controversy
Table of Contents
Dr. Geier's Research and Methodology
Dr. David Geier's research primarily focused on potential links between the MMR vaccine and autism, as well as the preservative thimerosal and developmental issues in children. While his work garnered significant attention, it also faced intense scrutiny due to methodological flaws. These flaws have been widely cited as reasons to question the validity of his conclusions.
-
Small sample sizes: Many of Geier's studies utilized small sample sizes, making it difficult to draw statistically significant conclusions about the population as a whole. Small sample sizes increase the likelihood of random variation influencing results and limit the generalizability of findings.
-
Lack of control groups: The absence of appropriate control groups in several studies hampered the ability to isolate the effects of vaccination from other potential contributing factors. This makes it difficult to determine causality.
-
Confounding variables not adequately addressed: Dr. Geier's research has been criticized for not adequately accounting for confounding variables, such as genetic predispositions, environmental factors, and pre-existing health conditions, which could influence the outcomes observed. These variables could easily skew the results and lead to erroneous conclusions.
-
Publication bias concerns: Concerns have been raised regarding potential publication bias, with critics suggesting that negative or inconclusive findings may not have been published, thus skewing the overall body of evidence. This selective reporting of data undermines the integrity of the research.
-
Questionable statistical analysis: The statistical methods employed in some of Geier's studies have been questioned by experts, raising concerns about the accuracy and reliability of the reported results. Improper statistical analysis can lead to spurious correlations and incorrect inferences.
It's important to note that many peer-reviewed publications have directly criticized the methodology employed in Dr. Geier's studies, highlighting these limitations and questioning the validity of his conclusions. Unfortunately, specific citations are difficult to provide comprehensively due to the dispersed nature of the critical analyses. However, a search for "critique of Geier vaccine studies" within academic databases will yield relevant results.
The HHS Response and Controversy
The HHS responded to Dr. Geier's research with investigations, sanctions, and retractions of some of his publications. This response itself became a source of significant controversy.
-
Accusations of censorship or suppression of dissenting views: Critics argued that the HHS response constituted censorship or suppression of dissenting views, hindering open scientific discourse on vaccine safety. This accusation fuels concerns about the potential limitations of free inquiry in sensitive areas of public health.
-
Debate regarding the appropriate level of scrutiny for vaccine safety research: The controversy sparked a wider debate on the appropriate level of scrutiny for vaccine safety research, highlighting the tension between the need for rigorous scientific standards and the potential for chilling effects on legitimate research.
-
Concerns about potential conflicts of interest: Concerns were raised regarding potential conflicts of interest within the HHS and its response to Geier's research, fueling accusations of bias and influencing the investigation's conclusions. Transparency regarding funding and affiliations is crucial in maintaining public trust.
-
Public perception and impact on vaccine hesitancy: The controversy surrounding Dr. Geier's research and the HHS response had a significant impact on public perception of vaccines and contributed to vaccine hesitancy. Misinformation surrounding vaccine safety can have severe consequences for public health.
Access to official statements from the HHS regarding this matter, along with relevant news articles and court documents, is readily available through a simple online search, though compiling a complete list here would be impractical.
Alternative Perspectives and Counterarguments
Large-scale, well-designed epidemiological studies consistently fail to find a causal link between the MMR vaccine and autism or between thimerosal and developmental issues. These studies, employing rigorous methodologies and significantly larger sample sizes, offer a counterpoint to Dr. Geier's research.
-
Overwhelming scientific consensus on vaccine safety: The overwhelming scientific consensus supports the safety and efficacy of vaccines. Numerous reputable organizations have affirmed this position, providing strong evidence-based support for widespread vaccination.
-
Explanation of established methodology for vaccine safety research: Established methodologies for vaccine safety research emphasize rigorous study design, large sample sizes, control groups, and appropriate statistical analyses to minimize bias and ensure reliable results. These methodologies are a cornerstone of scientific integrity.
-
Addressing common misconceptions about vaccines: It is crucial to address common misconceptions about vaccines, such as the false claims linking them to autism or other serious health problems. Providing accurate information is critical for combating vaccine hesitancy and protecting public health.
Reputable sources such as the CDC (Centers for Disease Control and Prevention) and WHO (World Health Organization) provide comprehensive information on vaccine safety and efficacy, offering a strong counter-narrative to the claims made in Dr. Geier's studies.
The Importance of Evidence-Based Medicine
Relying on robust, peer-reviewed research is paramount when assessing vaccine safety. Evidence-based medicine emphasizes the use of high-quality evidence to guide clinical decision-making. The David Geier vaccine study controversy highlights the dangers of misinformation and its potential impact on public health. Unfounded claims can erode trust in vaccination programs, leading to decreased vaccination rates and increased susceptibility to preventable diseases.
Conclusion
Dr. Geier's research faced significant methodological criticism, leading to controversy regarding its handling by the HHS. The scientific consensus overwhelmingly supports the safety and efficacy of vaccines. The debate highlights the importance of rigorous methodology and the dangers of spreading misinformation about vaccines. Understanding the nuances of the David Geier's Vaccine Study controversy is essential for informed decision-making about vaccination. Continue researching reputable sources to stay informed about vaccine safety and efficacy, and consult with your healthcare provider to address any concerns about vaccines. Critically evaluate information related to David Geier's Vaccine Study and other vaccine-related research to promote informed decisions about public health.

Featured Posts
-
 Whitecaps Stadium Talks Potential New Home At Pne Fairgrounds
Apr 27, 2025
Whitecaps Stadium Talks Potential New Home At Pne Fairgrounds
Apr 27, 2025 -
 Ecbs Initiative A New Task Force For Simplified Banking Rules
Apr 27, 2025
Ecbs Initiative A New Task Force For Simplified Banking Rules
Apr 27, 2025 -
 Los Angeles Wildfires The Unexpected Gambling Market
Apr 27, 2025
Los Angeles Wildfires The Unexpected Gambling Market
Apr 27, 2025 -
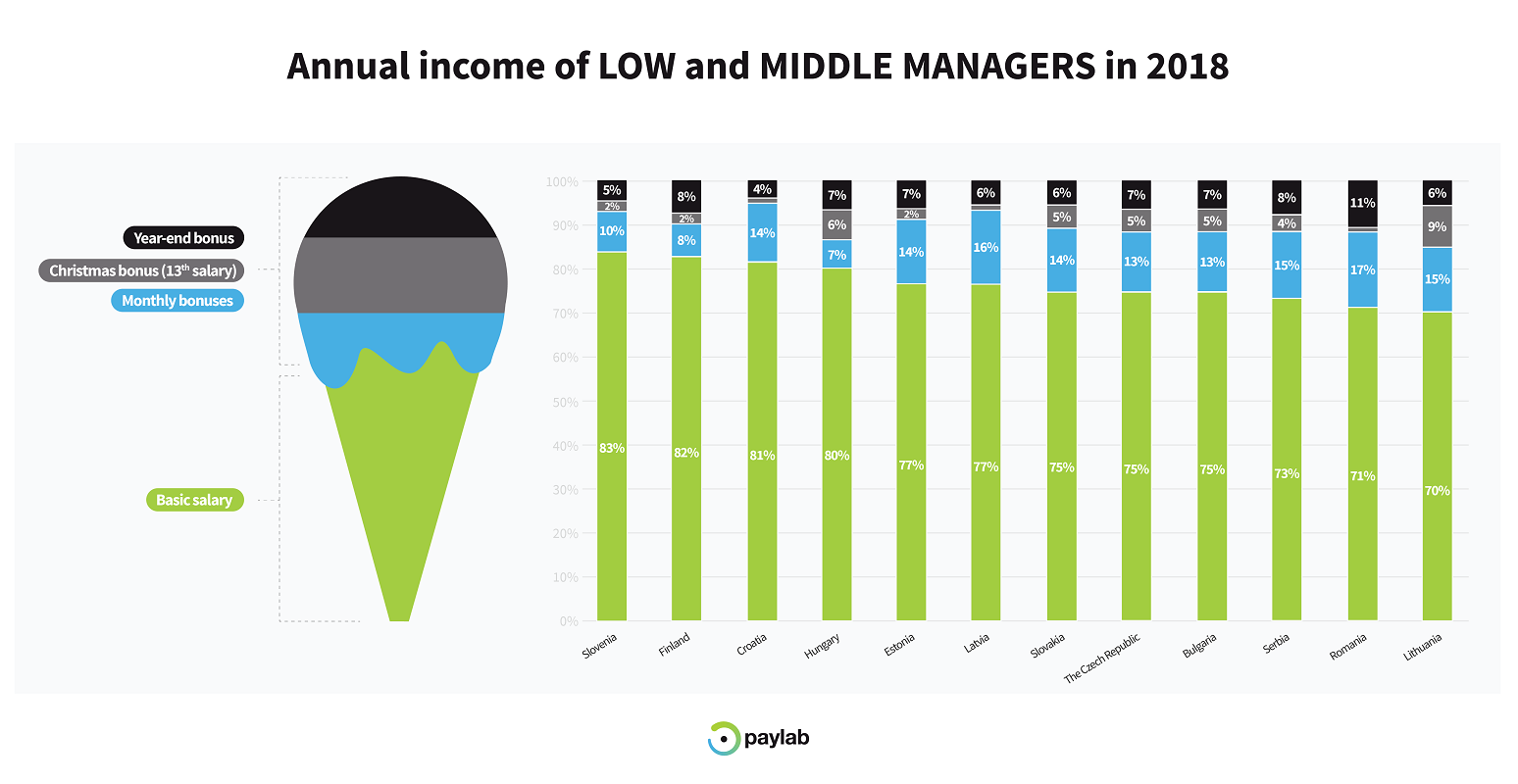 Understanding The Value Of Middle Managers Benefits For Companies And Employees Alike
Apr 27, 2025
Understanding The Value Of Middle Managers Benefits For Companies And Employees Alike
Apr 27, 2025 -
 Canadian Auto Industry Facing Posthaste Job Losses Due To Trumps Escalating Trade War
Apr 27, 2025
Canadian Auto Industry Facing Posthaste Job Losses Due To Trumps Escalating Trade War
Apr 27, 2025
